Portal Hypertension
William J. Cochran
The portal vein is formed at the junction of the superior mesenteric vein and the splenic vein. The inferior mesenteric vein joins with either the splenic or superior mesenteric vein. The portal vein goes to the hilum of the liver, where it bifurcates into right and left branches. These vessels continue to bifurcate until forming the hepatic sinusoids. The hepatic sinusoids then coalesce to form the hepatic vein, which ultimately joins the inferior vena cava.
Portal hypertension is an abnormal condition of sustained elevated pressure in the portal venous system. Normal portal vein pressure is between 5 and 10 mm Hg. Portal vein measurements rarely are made in clinical practice, because the methods for obtaining them are difficult and invasive. Several studies have documented that the complications of portal hypertension do not occur until the portal pressure gradient (i.e., the pressure gradient between the portal vein and the hepatic vein on the inferior vena cava) exceeds 10 to 12 mm Hg. Normal is less than 5 mm Hg. Above this threshold value, the absolute portal pressure correlates poorly with the complications associated with portal hypertension. The portal and hepatic veins do not have valves; therefore, the increased portal pressure results in increased blood flow and simultaneously increased pressure in the splanchnic system. This condition prompts the formation of portosystemic collaterals, which divert portal blood to the systemic circulation. In severe cases of cirrhosis, as much as 90% of the portal blood enters the systemic circulation through these collaterals and bypasses the liver.
Portal hypertension occurs more frequently in adult patients than in pediatric patients. Because adults are more frequently affected and clinical studies are more difficult to perform in the pediatric population, most of our knowledge regarding the etiology and treatment of portal hypertension comes from adult studies and from animal experiments.
ETIOLOGY
The two major factors that contribute to the development and maintenance of portal hypertension are increased vascular resistance and increased splanchnic blood flow. Increased vascular resistance to portal blood flow is the initiating factor responsible for the development of portal hypertension.
Three major sites of increased vascular resistance are seen: prehepatic, intrahepatic, and posthepatic (Box 371.1). Prehepatic portal hypertension is secondary to obstruction of portal venous flow. Within the prehepatic category, portal vein
thrombosis is the most common cause of portal hypertension in the pediatric population. Portal vein thrombosis can develop as a result of sepsis, pancreatitis, dehydration, shock, hypercoagulable states, umbilical vein catheterization, or omphalitis. Over half of the cases, however, are idiopathic. Other congenital anomalies are noted in 40% of the idiopathic cases compared with 12% of those with an identified postnatal cause. When this condition evolves, many small collateral veins develop to transport portal blood around the thrombosed portal vein to the liver. This condition is known as cavernomatous transformation of the portal vein, in which the normal portal vein is replaced by many small tortuous veins. Despite the presence of portal hypertension in these patients, their liver function studies and liver enzymes are normal. Patients with cavernomatous transformation of the portal vein can present at any age with splenomegaly or variceal bleeding or both. Classically, the frequency of variceal bleeding in this disorder was considered to decrease after adolescence, but more recent studies indicate that this is not the case. Variceal bleeding secondary to portal vein thrombosis can occur at any age and can result in massive, life-threatening bleeding.
thrombosis is the most common cause of portal hypertension in the pediatric population. Portal vein thrombosis can develop as a result of sepsis, pancreatitis, dehydration, shock, hypercoagulable states, umbilical vein catheterization, or omphalitis. Over half of the cases, however, are idiopathic. Other congenital anomalies are noted in 40% of the idiopathic cases compared with 12% of those with an identified postnatal cause. When this condition evolves, many small collateral veins develop to transport portal blood around the thrombosed portal vein to the liver. This condition is known as cavernomatous transformation of the portal vein, in which the normal portal vein is replaced by many small tortuous veins. Despite the presence of portal hypertension in these patients, their liver function studies and liver enzymes are normal. Patients with cavernomatous transformation of the portal vein can present at any age with splenomegaly or variceal bleeding or both. Classically, the frequency of variceal bleeding in this disorder was considered to decrease after adolescence, but more recent studies indicate that this is not the case. Variceal bleeding secondary to portal vein thrombosis can occur at any age and can result in massive, life-threatening bleeding.
BOX 371.1 Causes of Portal Hypertension
Prehepatic origin
Portal vein thrombosis
Intrahepatic origin
Presinusoidal
Schistosomiasis
Neoplasms
Hepatic cysts
Sinusoidal
Cirrhosis
Postsinusoidal
Venoocclusive disease
Posthepatic origin
Thrombosis
Budd-Chiari syndrome
Cardiac disease
Right-sided heart failure
Constrictive pericarditis
Increased vascular resistance in intrahepatic portal hypertension is secondary to increased intrahepatic and portocollateral resistance. Two major components of this increased intrahepatic vascular resistance are an irreversible component due to anatomic alterations and a reversible component that results from an increase in vascular tone.
The sites of anatomic abnormalities in intrahepatic portal hypertension can be divided into three major groups: presinusoidal, sinusoidal, and postsinusoidal. Hepatic schistosomiasis is the most common cause of presinusoidal portal hypertension worldwide, although it is exceedingly rare in North America. Patients with hepatic schistosomiasis develop portal hypertension as a result of ova deposition in the portal venules and the subsequent periportal granulomatous reaction. Overall, hepatic schistosomiasis is second only to cirrhosis as the most common cause of portal hypertension. Neoplasms and hepatic cysts, as seen in polycystic disease or Caroli disease, may compress the portal venules and result in presinusoidal portal hypertension.
The primary cause of sinusoidal portal hypertension in pediatric and adult patients is cirrhosis. As noted previously, cirrhosis is the most common cause of portal hypertension in adult and pediatric patients. Numerous pediatric disorders can lead to cirrhosis, in part because the liver responds to injury in a limited manner and cirrhosis is the final common pathway. The most common cause of cirrhosis in the pediatric population is biliary atresia, but many other disorders are associated with cirrhosis. These include alpha-1-antitrypsin deficiency, cystic fibrosis, infectious hepatitis, autoimmune hepatitis, and other metabolic disorders (see Chapter 370).
Venoocclusive disease is an example of a disorder causing intrahepatic postsinusoidal portal hypertension. Histologically, venoocclusive disease is characterized by sclerosis of the terminal hepatic veins, which results in increased resistance and the subsequent development of portal hypertension. This condition is relatively uncommon in children but occurs most frequently after bone marrow transplantation or in patients with immune deficiency. The risk that bone marrow transplant patients will develop venoocclusive disease is increased when leukemia is the reason for transplantation, when preexisting hepatic dysfunction is present, and when the procedure is the second bone marrow transplantation.
The classic cause of posthepatic portal hypertension is the Budd-Chiari syndrome, which is a thrombus in the hepatic vein at the entry to the inferior vena cava. Posthepatic portal hypertension also can develop as a result of severe right heart disease or constrictive pericarditis.
Anatomic changes are the most important component (the irreversible component) of increased intrahepatic resistance. The reversible component of increased hepatic resistance, increased intrahepatic vascular tone, has been shown to be present in patients with chronic liver disease and portal hypertension. Two cell types are involved: stellate cells and sinusoidal endothelial cells. Stellate cells (also called Ito cells) surround the sinusoidal endothelial cells. When injured, these cells produce collagen as well as smooth-muscle–like protein; the latter can contract when exposed to a number of substances, such as endothelin, angiotensin II, substance P, thrombin, and thromboxane. Current evidence supports the theory that, with injury, these stellate cells produce smooth-muscle actin, which results in perisinusoidal contraction and thereby alters sinusoidal blood flow.
The sinusoidal endothelial cells also respond to various vasoactive substances. Alterations in vasoactive compounds contribute to the reversible component of this increased intrahepatic vascular resistance. Evidence indicates that several vasoconstrictors including endothelin, angiotensin, norepinephrine, and vasopressin are increased in those with cirrhosis. Simultaneously, there is a reduction in several vasodilators including nitric oxide. These changes then result in increased vascular tone of the sinusoidal endothelial cells, thus contributing to the increased intrahepatic resistance.
CLINICAL MANIFESTATIONS
Portal hypertension can present as gastrointestinal (GI) bleeding, splenomegaly, ascites, or prominent abdominal vasculature. GI bleeding is frequently the presenting manifestation of portal hypertension and can occur as early as infancy. The bleeding is most frequently from esophageal varices but can occur from gastric, duodenal, or colonic varices. Rectal hemorrhoids are very uncommon in infants and young children; the presence of rectal hemorrhoids in this population should suggest portal hypertension. Portal gastropathy and portal colopathy are other sources of GI bleeding in patients with portal hypertension.
Splenomegaly is the second most frequent mode of presentation. These patients may present with splenomegaly or with hypersplenism. Most patients with portal hypertension eventually
develop splenomegaly, although no direct correlation is found between spleen size and the portal pressure gradient. The presence of upper GI bleeding in a patient with splenomegaly should be considered due to portal hypertension until proven otherwise.
develop splenomegaly, although no direct correlation is found between spleen size and the portal pressure gradient. The presence of upper GI bleeding in a patient with splenomegaly should be considered due to portal hypertension until proven otherwise.
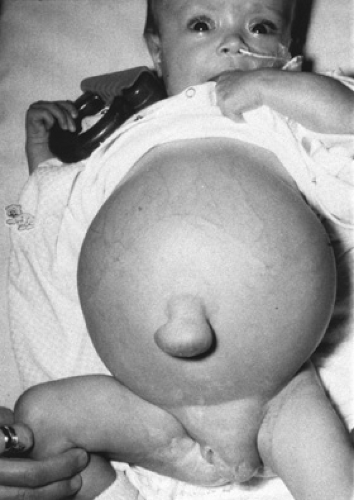 FIGURE 371.1. Female infant with cirrhosis due to biliary atresia. Note abdominal distension, umbilical hernia, and labial swelling due to massive ascites. |
Ascites is frequently a problem in patients with sinusoidal and postsinusoidal portal hypertension but is uncommon in patients with presinusoidal hypertension. Ascites may be minimal, detected only incidentally on ultrasound examination of the abdomen, or it may be massive and be associated with an umbilical hernia, labial or scrotal enlargement, or respiratory insufficiency (Fig. 371.1).
Much less commonly, portal hypertension presents with prominent abdominal vasculature. The prominence of abdominal vasculature is the result of diversion of portal blood, as in the case of varices. When these vessels radiate from the umbilicus, the condition is known as caput medusa.
DIAGNOSIS
The existence of portal hypertension can be determined by several modalities (Box 371.2), but the condition is diagnosed most frequently by physical examination. The most common physical manifestations of portal hypertension are splenomegaly, ascites, prominent abdominal vasculature, and hemorrhoids or rectal varices. When portal hypertension is secondary to chronic liver disease, other physical manifestations may be present, including icterus, a firm to hard liver, asterixis, spider hemangiomas, palmar erythema, encephalopathy, and malnutrition.
BOX 371.2 Diagnosis of Portal Hypertension
Physical examination
Splenomegaly
Ascites
Prominent abdominal vessels
Esophageal varices
Hemorrhoids/rectal varices
Evidence of chronic liver disease
Noninvasive techniques
Barium swallow
Ultrasonography
Invasive techniques
Endoscopy
Direct measurement of portal pressure
Measurement of hepatic venous pressure gradient
Angiography
Splenoportography
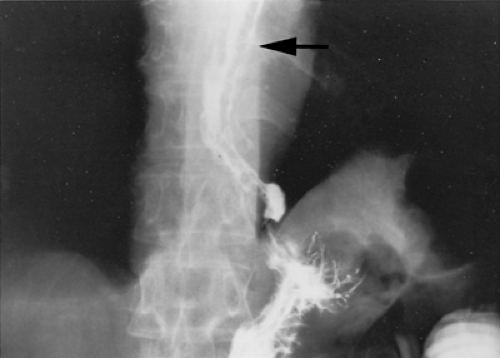
Several invasive and noninvasive techniques can be used to document portal hypertension. The two major noninvasive techniques are the barium swallow test and ultrasonography. Before the advent of flexible endoscopy, the barium swallow was the test performed most commonly to detect portal hypertension and its major complication, esophageal varices. The majority of patients with long-standing portal hypertension have esophageal varices, which can be identified by barium swallow as worm-like structures in the esophagus (Fig. 371.2). Ultrasonography is extremely useful in evaluating children with portal hypertension. In addition to predicting the presence of portal hypertension, ultrasonography is helpful in evaluating causes such as cirrhosis, hepatic cysts, or portal vein thrombosis. Ultrasonography also can assess spleen size, detect the presence of ascites, and determine if any associated renal abnormalities exist, such as are noted in congenital hepatic fibrosis. A classic ultrasonographic finding of portal hypertension in adult patients is an enlarged portal vein diameter. The portal vein also should be assessed in relation to respiration. In normal patients, the portal vein increases in diameter with inspiration. This increase with inspiration does not occur in patients with portal hypertension, and its absence may be a more reliable indicator of portal hypertension than is the actual diameter of the portal vein. A more reliable marker of portal hypertension in pediatric patients is the ratio of portal vein diameter (in millimeters) to body surface area (in square meters). If this ratio
exceeds 12, esophageal varices are likely. Another parameter is the ratio of the lesser omentum thickness to aortic diameter. In patients with portal hypertension, blood flow through the lesser omentum rises, increasing its thickness and thus increasing this ratio. A ratio of greater than 1.9 is a good predictor of the presence of esophageal varices.
exceeds 12, esophageal varices are likely. Another parameter is the ratio of the lesser omentum thickness to aortic diameter. In patients with portal hypertension, blood flow through the lesser omentum rises, increasing its thickness and thus increasing this ratio. A ratio of greater than 1.9 is a good predictor of the presence of esophageal varices.
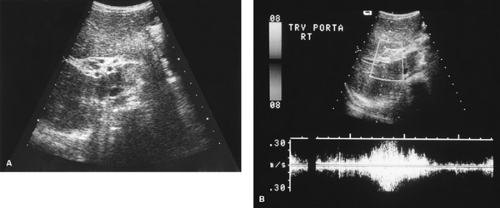 FIGURE 371.3. A: Ultrasonic examination of patient with cavernomatous transformation of the portal vein demonstrating the small collateral veins. B: Doppler ultrasonographic study of the same patient; the collateral veins are enclosed in the rectangle. See Color Figure 371.3B in color section; the collateral veins appear blue in that image. |
Doppler ultrasonography can be used to assess blood flow within the portal vein, which normally is 10 to 30 mL/second and hepatopetal in direction. Because of increasing vascular resistance, blood flow decreases as portal hypertension increases and, in severe cases, the direction of the blood flow may be reversed (hepatofugal flow). In cases of cavernomatous transformation of the portal vein, color Doppler ultrasonography can help to determine the presence of small collaterals around the obstructed portal vein (Fig. 371.3).
Other studies using Doppler ultrasonography in children suggest that portal vein pulsatility is a sensitive and specific finding indicative of portal hypertension in children. Another potentially useful parameter is maximal velocity of the main portal vein. This value decreases as the severity of liver disease increases.
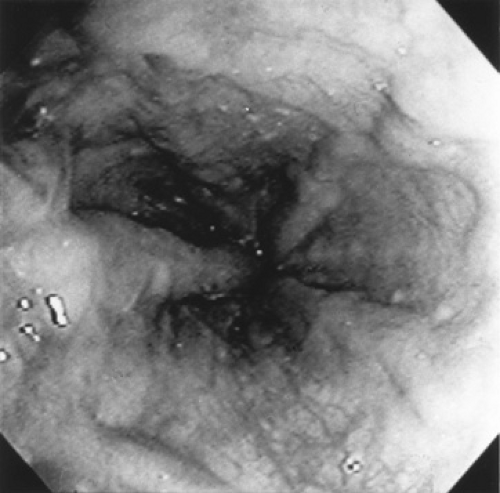
Invasive techniques to determine the presence of portal hypertension include endoscopy, direct measurement of portal pressure, measurement of hepatic venous pressure gradient, angiography, contrast-enhanced computed topography, magnetic resonance angiography, and splenoportography. Endoscopy can be performed safely in pediatric patients and is more sensitive in detecting esophageal varices than is the barium swallow test or ultrasonography. Endoscopy allows visual inspection of the varices to determine size and color, which is helpful in predicting the risk of bleeding (Fig. 371.4). Endoscopy can also determine the presence of portal gastropathy and gastric and duodenal varices.
Portal pressure can be measured directly by percutaneously puncturing an intrahepatic branch of the portal vein or during abdominal surgery by inserting a needle directly into the portal vein. The former procedure is difficult to perform in pediatric patients, and the latter procedure is unacceptable for the sole purpose of diagnosing portal hypertension. Direct measurements are most commonly performed in a research setting.
The hepatic venous pressure gradient is measured by placing a catheter in the hepatic vein under fluoroscopic control. The free hepatic venous pressure is obtained, and the catheter is advanced until the catheter occludes a small hepatic vein. The pressure is obtained in this position and is known as the wedged hepatic venous pressure. The hepatic venous pressure gradient is the difference between the wedged hepatic venous pressure and the free hepatic venous pressure. This gradient is normally less than 5 mm Hg; a value greater than 10 mm Hg is indicative of portal hypertension. Complications of portal hypertension typically do not occur until the hepatic venous pressure gradient exceeds 12 mm Hg. Patients with prehepatic portal hypertension, as in portal vein thrombosis, have a normal hepatic venous pressure gradient. Pressure gradient measurement is also invasive and is most commonly used in adult studies assessing the efficacy of pharmacologic agents to reduce portal pressure.
The major role of angiography in patients with portal hypertension is to rule out vascular thrombosis, as in the Budd-Chiari syndrome or portal vein thrombosis, and to define the vascular anatomy if surgery is contemplated. Recent advances in contrast-enhanced computed topography and magnetic resonance angiography have limited the use of mesenteric angiography.
Splenoportography is performed by the direct puncture of the spleen percutaneously. Because of the high risk associated with this procedure and because the portal system can be visualized with less invasive techniques, it is rarely performed. The procedure should not be used unless surgery can be performed immediately should complications occur.
Various laboratory studies are important in patients with portal hypertension—not to diagnose the condition but to further assess hepatic function and the patient’s nutritional status and to evaluate for the presence of hypersplenism. This laboratory evaluation should consist of a complete blood count and measurement of serum electrolytes, coagulation studies, and liver and renal profiles.
BOX 371.3 Complications of Portal Hypertension
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree