Malignant Brain Tumors
Murali M. Chintagumpala
Primary brain tumors are the second most common type of cancer reported in children and adolescents. In the United States, the annual incidence of primary brain tumors in children younger than 15 years is approximately 1,200 new cases each year. Unfortunately, progress in the field of pediatric neurooncology has been slow compared with that in other childhood malignancies.
Surgery and radiation therapy traditionally constituted the standard therapeutic approach. The tendency has been to manage these children within the general medical community, contrary to the now common practice of referral to a pediatric cancer center for definitive diagnosis and treatment. Pessimism has followed failure to excise the tumor completely, and chemotherapy has been viewed as noxious, with little justification. However, progress in the comprehensive care of children with malignant brain tumors under the direction of experienced pediatric neuro-oncology teams has provided a rational basis for a departure from a previously gloomy scenario. Tumor heterogeneity, the relatively small numbers of patients with a specific tumor type available for investigation, the limitations of drug delivery across the blood–brain barrier, and the relative biologic resistance to therapy in certain tumors, such as gliomas, are unique technical and theoretical challenges that can be resolved only by continued collaborative clinical and laboratory research.
CLINICAL MANIFESTATIONS AND COMPLICATIONS
Early symptoms of central nervous system (CNS) tumors frequently are nonspecific. In infants with open sutures, these may consist of increased head circumference, irritability, head tilt, and loss of developmental milestones. Older children may present with headache. This symptom usually increases in frequency, becomes more severe in the morning, and is typically followed by vomiting. Approximately 85% of the children with malignant brain tumors have abnormal findings on neurologic or ocular examinations within 2 to 4 months of the onset of headaches.
Children who report an unchanging pattern of headaches without focal neurologic findings for more than 12 months have a low probability for CNS tumors. Specific neurologic symptoms such as ataxia, somnolence, hemiparesis, seizures, head tilt, cranial nerve palsies, diencephalic syndrome, and diabetes insipidus may occur later in the illness and may suggest localization of the CNS tumor.
The differential diagnosis for CNS tumors in children is extensive and includes brain abscesses, hemorrhage, nonneoplastic hydrocephalus of any cause, arteriovenous malformations or aneurysm, and indolent virus infections.
Classification
Traditionally, CNS tumors of childhood have been classified on the basis of location (e.g., infratentorial versus supratentorial) and histology. In children between the ages of 4 and 11 years, infratentorial (posterior fossa) tumors predominate. These include cerebellar tumors and brainstem tumors. Supratentorial tumors occur more frequently during the first years of life and during late adolescence and young adulthood. Approximately 45% of the childhood brain tumors arise in the cerebellum. Cerebellar astrocytomas and medulloblastomas are the tumors diagnosed most frequently in this region. Ependymomas that arise in and around the fourth ventricle represent between 3% and 14% of all childhood tumors and have been included as cerebellar tumors by some authorities.
The cerebrum is the next most common site of involvement in children, accounting for 20% to 27% of all brain tumors. The most frequent tumors include astrocytomas, glioblastomas, and ependymomas. Brainstem neoplasms account for 9% to 15% of all intracranial neoplasms. Approximately 75% of all brainstem tumors occur in children younger than 10 years. Midline tumors, which include a mix of germ-cell
tumors, craniopharyngiomas, pinealomas, optic gliomas, and pituitary adenomas, account for another 10%.
tumors, craniopharyngiomas, pinealomas, optic gliomas, and pituitary adenomas, account for another 10%.
The traditional practice of classifying CNS tumors on the basis of location is being reevaluated. An international panel of neuropathologists has proposed a revision of the World Health Organization (WHO) classification system to classify tumors on the basis of histopathologic features alone. For example, medulloblastoma, a highly malignant, poorly differentiated, “small, blue, round cell” tumor was said to arise only within the cerebellum. The revised classification system recognizes this tumor as a primitive neuroectodermal tumor (PNET), with or without elements of astrocytic, neuronal, or ependymal differentiation. Tumors of this identical histologic type arising anywhere within the brain are classified as PNETs. The pinealoblastoma arising in the pineal region, or the ependymoblastoma or classic PNET arising within the supratentorial regions, are identified by the new classification schema as PNETs. However “medulloblastoma” is still retained for PNET of the cerebellum. PNETs arising from different areas of the brain display similar histology but appear to be biologically different, with different cytogenetic abnormalities and different outcomes when treated in a similar manner. This classification system recognizes the heterogeneity of tumors arising within a single site, and it may be useful for future prognostic staging and for the design of new therapeutic strategies.
DIAGNOSIS
Computed tomographic (CT) scanning, with and without contrast enhancement, had been the standard noninvasive diagnostic tool. The unenhanced CT scan can suggest whether a lesion is cystic or solid and whether calcifications, hemorrhage, edema, and hydrocephalus exist. After intravenous contrast, enhancement of the tumor occurs because of a disruption of the blood–brain barrier. This improves detection of small tumors, definition of isodense or hypodense regions within the tumor, and differentiation of areas of edema surrounding the tumor mass. Subarachnoid and leptomeningeal seeding of tumor also may be detected with enhanced scans. Cranial CT scans have a sensitivity of greater than 94% for primary brain tumors, but certain limitations of resolution must be recognized. Small lesions within the posterior fossa, especially within the brainstem, and small midline cystic structures near the base of the skull occasionally escape detection.
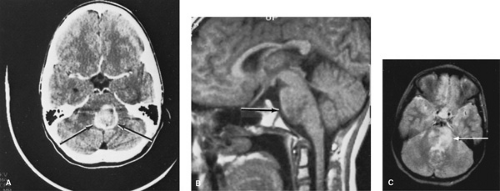
Magnetic resonance imaging (MRI) with gadolinium enhancement is a sensitive neuroimaging technology for the diagnosis of CNS tumors and is now perceived as the standard diagnostic modality in the diagnosis and management of primary brain tumors. MRI scans are superior to CT scans in the detection and definition of low-grade glial tumors and of lesions at the vertex, within the posterior fossa (especially within the brainstem), near the wall of the middle fossa, and at the base of the skull (Fig. 306.1). MRI myelogram with gadolinium enhancement is the best method for detecting spinal cord tumors or delineating leptomeningeal tumor invasion. MRI scans use no ionizing radiation and have no calvarium artifact. Limitations include increased cost, longer scan times and duration of sedation in the young patient, an inability to detect calcifications, and limited access to the patient during the actual scan time.
The child with a positive scan result may benefit from additional diagnostic procedures. Angiography gives information about blood supply and may occasionally assist the neurosurgeon in planning the operative approach. Cerebrospinal fluid (CSF) examination is of great importance in patients presenting with PNETs, germinomas, ependymomas, and high-grade glial tumors, but it must be performed with great care in patients with increased intracranial pressure. Lumbar puncture for CSF may be safely performed 14 to 21 days after the initial operation and intracranial decompression. When CSF is obtained immediately after surgery for the primary tumor, it appears to be of limited value. Standard radionuclide scans no longer are used routinely. Spectroscopy, perfusion/diffusion studies, and positron emission tomography (PET) studies have the potential to provide additional information with respect to diagnosis and management of these children with brain tumors. These studies may help differentiate viable from necrotic tumor after therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree